Streptococcus suis – a strong negative economic impact on the swine industry
Streptococcus suis (S. suis) was first reported in 1954 after outbreaks of meningitis, septicemia, and purulent arthritis among piglets (Hughes et al., 2009). Nowadays, it can still be seen as a major porcine pathogen. Infection with S. suis is one of the main causes of death during the post-weaning period from 5 to 10 weeks of age (Segura et al., 2016). The disease has a strong negative economic impact on the swine industry. It leads to increased medication (prevention & treatment), mortality, and decreased performance. According to Napp et al. (2021), in Germany, the losses per pig due to S. suis at the end of the production cycle can reach € 2.28, in the Netherlands - €1.54 and in Spain - €0.96 respectively.
The pathogen
Streptococcus suis is an encapsulated, facultative anaerobic, Gram-positive, α-hemolytic coccus. Over the years, 35 serotypes (1-34 and ½) have been identified based on taxonomic classification and capsule typing. Among them, serotype 2 is considered the most common cause of the disease in Europe and worldwide. Moreover, S. suis is an emerging zoonotic pathogen of humans who come in contact with infected pigs or consume pork products that get contaminated with this pathogenic bacterium. Serotype 2 is the major zoonotic agent (Gottschalk et al., 2014). S. suis can survive in various matters and conditions: faeces - 0°C: 104 days; 9°C: 10 days; 22-25°C: 8 days; dust - 0°C: 54 days; 9°C: 25 days; 22-25°C: > 24 h; water - 4°C: 1-2 weeks; 50°C: 120 min 60°C: 10 min. Bonifait et al. (2014) also isolated S. suis strains from aerosols inside swine confinement buildings. Commonly used disinfectants effectively kill S. suis in less than 1 minute.
|
Country |
Nursery |
Fattening |
|
Germany |
60.7% |
36.4% |
|
Netherlands |
55.7% |
53.0% |
|
Spain |
84.6% |
43.8% |
Table 1: Proportion of farms presenting S. suis infection per country per year by type of production site.
Almost 100% of pig farms worldwide are positive for S. suis, but not all farms report clinical cases. The report of IRTA on the incidence of S. suis infection in pigs in Spain, Germany, and The Netherlands (2020) demonstrates that the problems associated with S. suis are very common in the three countries, with the proportion of affected farms per year ranging between 56% and 85% for nursery, and between 36% and 53% for fattening units, with Spain as the country with the highest proportion of affected farms (Table 1).
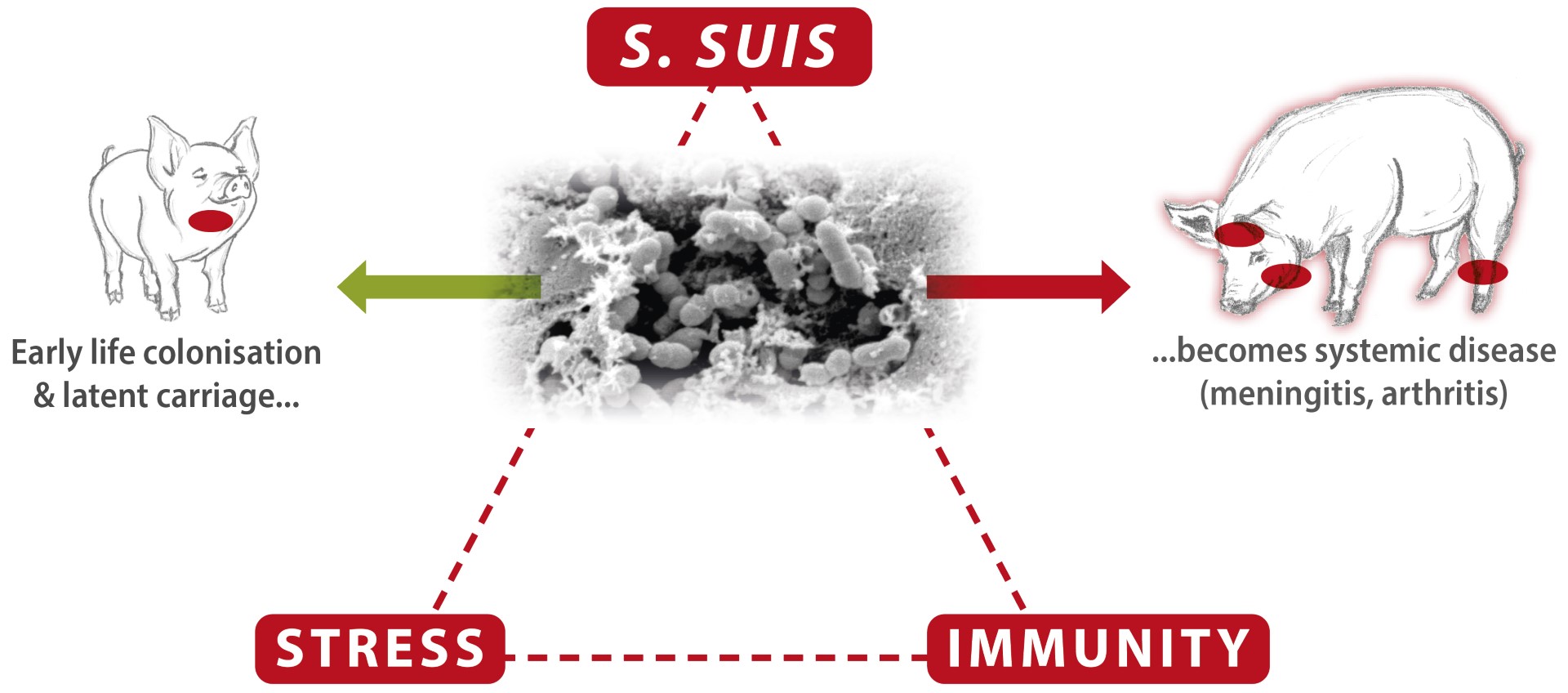
Early life infection & latent carriage
It is usually accepted that pigs’ main route of infection with S. suis is through the respiratory tract (oro-nasal) (Dekker et al., 2013). The pathogen is transferred from the sow’s vaginal secretions during parturition and the first days of life to the orо-nasal cavity of piglets and colonises their tonsils soon after birth (Gottschalk et al., 2003). Horizontal transmission can also be part of the infection through contact with other herd members, especially during outbreaks (Segura et al., 2016).
After early life colonisation, the pathogen remains in the tonsils, while the infected pigs remain latent carriers without being sick. Therefore, the bacterium is already present in the herd and is waiting for predisposing factors that decrease immunity and contribute to disease occurrence (Cloutier et al., 2003, Gottschalk & Segura, 2019).
The role of the immune status and stress is crucial for the outbreak of S. suis infection
Fig. 1 Interaction between S.suis, stress level and immune status is crucial for developing systemic S. suis disease.
As S. suis is a facultative pathogen, different factors, both biotic (viral infections, for instance) and abiotic (such as weaning, ventilation problems and change of feed), can trigger its outbreaks (Seitz et al., 2016). The cumulating stress factors and inflammation during the pigs’ post-weaning/ transition period challenge the immunity mightily so that the threshold for keeping S. suis restricted can be exceeded. The pathogen penetrates mucosal barriers and occurs in the blood circulation, causing septicemia and organ dissemination, resulting in systemic disease, manifested with sudden mortality, central nervous symptoms (meningitis), joint problems (arthritis), and, less often, endocarditis and respiratory symptoms.
The morbidity of S. suis infections in pig herds rarely exceeds 5%, although it can reach over 50% in poorly managed farms and the presence of secondary diseases. When applying appropriate measures and treatment, the mortality usually remains low but can, at the same time, reach levels of up to 20% in untreated herds. (Dutkiewicz et al., 2017)
It is proven that S. suis outbreaks are exacerbated on farms positive to immunomodulatory viruses like PRRSv, and PCV2. The Swine influenza virus also contributes to the worsening of the situation. Hence, knowing the dynamics of these infections in each farm is very important.
The porcine stomach is a very efficient barrier against oro-gastrointestinal S. suis infection.
S. suis can be recovered from saliva (Dekker et al., 2013), but in vitro analyses demonstrate that S. suis fails to survive in the stomach contents of differently-fed growing piglets (Segura et al., 2016; Baums et al., 2016). The oral experimental infection by feeding piglets a diet mixed with a high dose of S. suis serotypes 2 or 9 did not lead to any detection of the orally applied challenge strains in lymph nodes or internal organs and resulted in the absence of clinical disease (Baums et al., 2016).
Increase of S. suis outbreaks after the EU ban on therapeutic levels of zinc oxide (ZnO)
As a result of restrictions on the use of ZnO, the number of cases of meningitis and polyarthritis related to S. suis are increased. This observation can be associated with increased intestinal inflammation during the post-weaning/ transition period without antimicrobial and ZnO treatment, especially if the feed recipes are not adapted to the physiology of the animals and legislative changes, respectively.
So far, the nutrient requirement estimates and the feed recipes are largely based on growth performance; however, their effects on intestinal development, immune challenges, and response require strong attention in post-antimicrobials and the ZnO era. It is well-known that increased intestinal permeability due to weakened tight junctions during weaning or sharp changes in the feed leads to the transmigration of luminal antigens, toxins, viruses or even bacteria into the lamina propria. This leads to the activation of the cells of the immune system that reside within the lamina propria and releases pro-inflammatory mediators and signalling molecules to help recruit other immune cells into the region (Lalles et al., 2007), i.e. the immune system is activated.
This challenge often appears crucial for S. suis outbreaks because it comes on top of other challenges and immune stimulation during the pigs' stressful post-weaning/transition period.
ZnO's immunomodulatory role (cytokine expression) and antioxidative efficacy might also be involved, but further investigations are needed.
Diagnosis and differential diagnosis
A presumptive diagnosis of S. suis infection is based on history, clinical signs, and gross lesions but requires laboratory confirmation. The proper sampling procedure is crucial to confirm or reject the diagnosis.
S. suis problems need to be differentiated from other pathologies related to nervous symptoms also typical for Glaesserella parasuis, Shiga-toxigenic Escherichia coli, sodium toxicity (high salt consumption, or water deprivation), phenylarsonic compound (organic arsenic) poisonings, but also Aujeszky disease etc.Also, differential diagnosis with Mycoplasma hyorhinis, Mycoplasma hyosynoviae, Glaesserella parasuis, Erysipelothrix rhusiopathiae, and other pathologies related to polyarthritis need to be performed. Additional differential diagnoses that need to be excluded are Mycoplasma hyorhinis, Mycoplasma hyosynoviae, Erysipelothrix rhusiopathiae, and other causes of polyarthritis.
Current strategies for control
The lack of an effective commercial vaccine to control and prevent S. suis infections and the controversial results of autogenous vaccines have promoted extensive prophylactic and metaphylactic treatments with a wide range of antimicrobial agents, such as penicillin, ampicillin, amoxicillin, or trimethoprim/sulfa, on farms (Varela et al. 2013; Seitz et al., 2016).
Despite the massive use of antimicrobials against S. suis and increased public health concerns about their resistance, some pigs become sick with often poor prognoses (Seitz et al., 2016).
Because of the specificity of the disease, S. suis requires prolonged antimicrobial treatment. In the field often, cases of lowering the doses can be observed. At the same time, sub-inhibitory concentrations of amoxicillin decrease its efficacy while increasing the S. suis inflammatory potential (Daniel Grenier et al., 2016).
According to the European Medicines Agency (EMA) Antimicrobial Expert Group (AMEG) Classification of WHO Critically Important Antimicrobials (CIAs) based on the level of risk to humans due to antimicrobial resistance development following use in animals, broad-spectrum penicillins are in the group with the highest risk to public health. European legislation strongly emphasised the reduction of antimicrobial usage in relation to antimicrobial resistance. In 2022, new EU legislation prohibited all forms of routine antibiotic use in farming, including preventative group treatments.
All of the above put the effective control of Streptococcus suis outbreaks under a strong challenge.
To reduce antimicrobial use, S. suis disease prevention should concentrate on managing predisposing factors (stress) in combination with proper alternative treatment targeting Gram+ bacteria (S. suis) and strong immunity support of the animals, especially during the stressful post-weaning/transition period.